Brain Disorder Translational Research Team
TEAM INTRODUCTION

Team Leader
Makoto HIGUCHI [ M.D., Ph.D. ]
The mission of the Neuromolecular Dynamics Team is to conduct neuroimaging research on animal models of neuropsychiatric illnesses in conjunction with multimodal technologies, including neuropathological, behavioral and electrophysiological analyses, leading to direct insights into early diagnosis and efficacious therapies of the diseases. In addition, our research projects are committed to elucidating molecular interactions that underlie visibility of neuroglial components in intravital neuroimaging.
RESEARCH INTRODUCTION
Translational research bidirectionally linking animal models and humans to elucidate molecular etiology of neuropsychiatric disorders.
-
1. Visualization of protein aggregates mechanistically implicated in neurodegenerative dementias
Alzheimer's disease is a representative dementing disorder, and is pathologically characterized by depositions of two proteins, amyloid-beta and tau, in the brain. In a pathogenetic condition, these proteins form fibrillary aggregates, which are intimately linked to neurodegenerative processes leading to neuronal death and cognitive impairments. Tau fibrils are also known to be deposited as core pathologies in diverse dementias of non-Alzheimer type. By developing PBB3, a PET imaging agent for tau aggregates, we provided the first successful demonstration of visualization of tau pathologies in the brains of living patients with Alzheimer's disease and allied disorders.
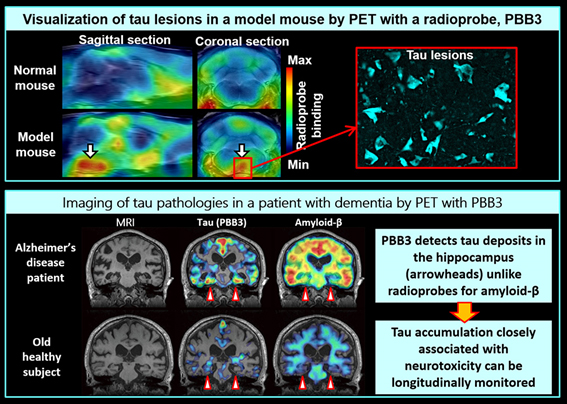
PET imaging of tau deposits was proven possible by scanning a mouse model of dementia developing tau pathologies in the brain immediately after intravenous injection of PBB3 into this animal. We subsequently applied PBB3 to human PET, and succeeded in capturing the accumulation of tau fibrils in Alzheimer's disease patients. These patients exhibit notable accumulation of PBB3-positive tau lesions in the hippocampus (arrowheads), which is critically involved in the memory function, By contrast, the deposition of PET-detectable amyloid-beta in the hippocampus is minimal. As MRI indicates remarkable hippocampal atrophy in the patients, it is presumable that tau deposits rather than amyloid-beta accumulations are essentially associated with local neuronal death.
Since PBB is also a fluorescent molecule, visualization of individual tau lesions in the brain of a mouse model of tau pathology has been enabled by performing two-photon laser microscopy of this animal after injection of non-radiolabeled PBB3. This technology allows real-time assessments of relationships between the formation of tau aggregates and neuronal death.
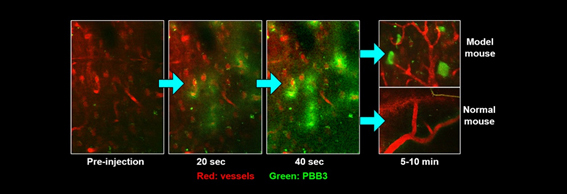
In vivo imaging of the mouse brain at a subcellular resolution is possible by two-photon laser microscopy. Blood vessels are labeled with a red dye, SR101. Intravenously injected PBB3 promptly enters the brain (visualized in green) through capillary vessels. In a mouse model of tau pathology, PBB3 binds to tau lesions in neurons and illuminates them. Thus, the kinetics of an imaging agent can be analyzed in a real-time manner. This technique also enables daily or weekly observations of the same cell and/or tau inclusion for the pursuit of the tau fibril formation and neuronal loss along the course of the tau pathogenesis.
We also reported the first PET imaging of amyloid-beta deposits in the brains of mice modeling amyloid-beta pathologies. By comparing PET images of brain amyloid in humans and mouse models, we revealed that PET-detectable amyloid lesions are primarily composed of a neurotoxic amyloid-beta subspecies.
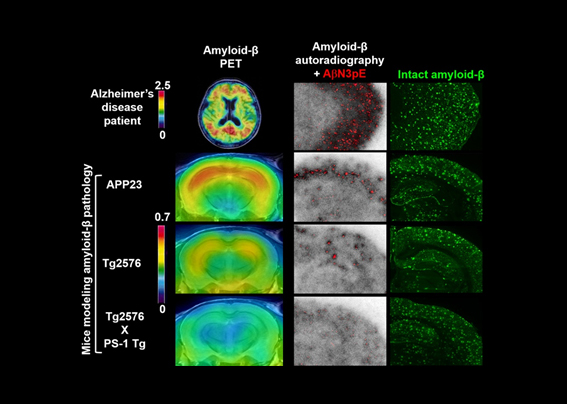
PET captures amyloid-beta deposits in the brain. Scale bars indicate amounts of a PET imaging agent, PiB, bound to amyloid-beta fibrils. Mouse models of amyloid-beta pathology develop highly abundant amyloid-beta deposits in the brain, while these animal strains display much less PET-detectable amyloid-beta than Alzheimer's disease patients. To clarify mechanisms underlying this discrepancy, autoradiography with PiB and immunostaining of brain sections were conducted, and demonstrated that the accumulation of a truncated and modified amyloid-beta subspecies, AbetaN3pE, is tightly associated with the formation of binding components for PiB, and exerts marked neurotoxicity.
-
2. Clarification of roles played by inflammatory and immunocompetent cells in the brains under neuropsychiatric conditions
Activation of microglia and some other glial subtypes responsible for immune responses in the brain is accompanied by expression of translocator protein (TSPO) in these cells. We developed a PET imaging agent for TSPO, and documented the first report of PET visualization of TSPO-positive inflammatory gliosis in mouse models of dementia. This resulted in a revelation that there exist two modes of microglial activation consisting of beneficial activation protecting neurons by eliminating misfolded amyloid-beta and tau and deleterious activation attacking neurons by accelerating depositions of amyloid-beta and tau and producing reactive oxygen species. The TSPO-PET imaging technique has been applied to humans to clarify roles of protective and aggressive microglial populations. Microglial activation is also implicated in psychiatric disorders such as depression, and we are accordingly promoting imaging studies connecting information on neuroinflammatory processes in patients with these illnesses and animal models.
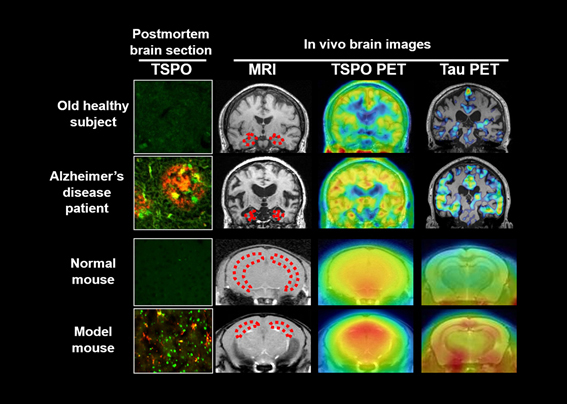
In the brain of an Alzheimer's disease patient, depositions of amyloid-beta and tau (red in brain sections) are accompanied by accumulations of TSPO-expressing detrimental microglia (green in brain sections). Such aggressive microglia also accumulate in the brain of a mouse model of tau pathology. It has been indicated by PET and MRI that the tau deposition, TSPO-positive microgliosis and atrophy of the hippocampus (outlined by dotted lines) and several other brain areas are closely and reciprocally related to one another.
-
3. Development of imaging technologies to investigate influences of neurotransmission abnormalities on brain functions
Diverse neurotransmission systems, including dopamine, serotonin, noradrenaline, glutamate and GABA transmissions, support brain activities at a molecular level, and are supposed to be dysregulated in neuropsychiatric disorders, giving rise to the symptomatic manifestations. We are currently analyzing the release of neurotransmitters and changes in the status of neuroreceptors by PET imaging of the receptors and transporters for the transmitters in rodent models and humans. Correspondingly, imaging of brain activities by two-photon laser microscopy and functional MRI and measurements of neurotransmitters by MR spectroscopy are performed to investigate impacts of altered neurotransmissions on the brain functions at cellular, circuit and global levels.
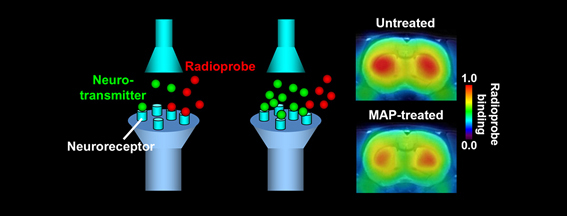
PET imaging of dopamine D2 receptors provides information on the endogenous dopamine neurotransmission. A PET ligand, MNPA, binds to D2 receptors, but this binding is displaced with an endogenous neurotransmitter, dopamine, when the release of dopamine into the synaptic cleft is enhanced. Reduced binding of MNPA to D2 receptors is observed by PET scans of the rat brain following administration of a psychostimulant, methamphetamine (MAP), indicating a surge of the dopamine concentration in the synaptic cleft induced by MAP.
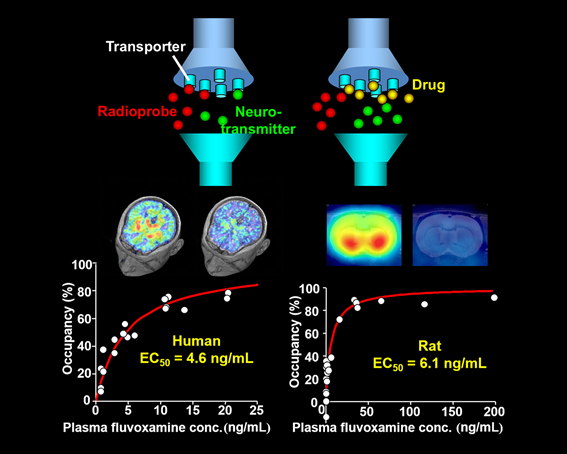
Actions of an anti-depressant, fluvoxamine, in the brains of humans and rats are visualized by PET imaging of serotonin transporters. A PET imaging agent, DASB, binds to serotonin transporters, but this binding is displaced with fluvoxamine in a manner dependent on the concentration of the drug in the brain and plasma. The reduction of DASB-PET signals accordingly indicates the occupancy of serotonin transporters by fluvoxamine. The plasma concentrations of fluvoxamine inducing 50% occupancy of serotonin transporters in the brains of humans and rats are similar, and therefore the dosage of fluvoxamine yielding therapeutic effects in humans can be predicted from rat data.